Abstract
Background: in vivo T-cell depletion (TCD) is frequently used in the setting of allogeneic hematopoietic stem cell in order to decrease acute and chronic GVHD incidence. Several randomized studies, enrolling patients with myeloid disease have concluded to a decreased risk of GVHD without increasing the relapse risk. The present study included only myelodysplastic syndrome patients and evaluated the impact of anti-thymoglobulin (ATG) or alemtuzumab on outcomes.
Method: these patients come from a Data Quality Initiative consisting in an improvement of the quality of the data asking centers to complete the missing data. 1284 patients transplanted in 14 centers between 2003 and 2014 have been included in this study. Median follow-up of the patients was 25 months. GRFS was defined as survival without relapse and without chronic extensive GVHD and no previous grade III-IV acute GVHD. Cytogenetics was defined according to classical IPSS and not calculated if patients were transformed into AML given 5 groups of patients: good, intermediate, poor, AML and missing (n=98). Multivariable models based on Cox proportional hazard were performed to test potential predictors and ATG effect was thus included in the models. Statistical analysis was performed using R software.
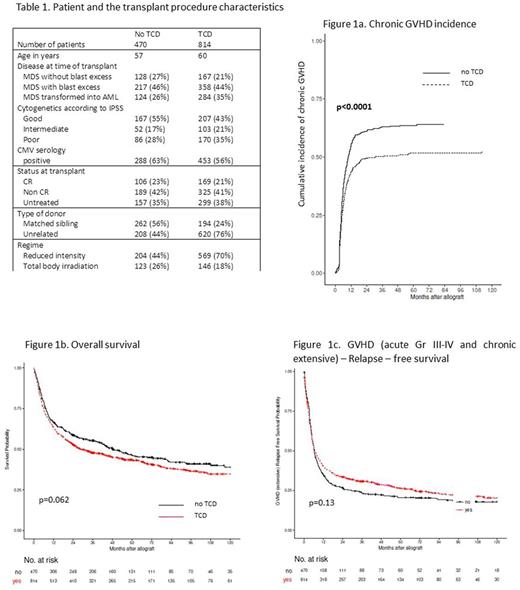
Results: 814 patients received TCD and 470 did not. Characteristics of patients and transplant according to the TCD is given in the Table 1. Among patients who received a TCD, 168 received alemtuzumab while the other patients received ATG, either Grafalon® or Thymoglobuline®. At 6 months, cumulative incidences of acute grade II-IV GVHD and grade III-IV GVHD were 33% vs 31% and 13% vs 13% without or with TCD. At 5 years, chronic and extensive GVHD incidences were 64% vs 52% (Figure 1a. Chronic GVHD incidence) and 38% vs 25% without or with TCD. At 5 year, relapse incidence, relapse-free survival (RFS), OS and GRFS were 23% vs 28%, 42% vs 39%, 47% vs 43%, and 21% vs 26% without or with TCD (Figure 1b: OS, Figure 1c: GRFS).
In the multivariable models for OS, adjusted on age (HR: 1.01, p<0.001), Karnofsky score (HR: 0.99; p<0.0001), disease classification (MDS without blast excess, HR: 0.7, p=0.004), disease status (noCR, HR: 1.3, p=0.002), platelet count at time of transplant (HR: 0.46/1 Giga, p=0.03), type of donor (unrelated, HR=0.99, p=0.94) and cytogenetics (intermediate, HR=1.01, p=0.92; poor, HR=1.5, p=0.004; AML, HR=1.3, p=0.01; unknown, HR=1.1, p=0.44), TCD had no significant impact (HR: 1.1, p=0.12).
In the multivariable models for GRFS, adjusted on age (HR: 1.01; p=0.003), Karnofsky score (HR: 0.99; p=0.0003), disease classification (MDS without blast excess, HR: 0.8, p=0.05), disease status (noCR, HR: 1.1, p=0.049), type of donor (unrelated, HR: 1.5, p=0.058) and cytogenetics (intermediate, HR=1.2, p=0.058; poor, HR=1.33, p=0.003; AML, HR=1.2, p=0.014; unknown, HR=1.2, p=0.21), TCD had a protective effect on GRFS (HR: 0.8, p=0.009).
In the multivariable models for relapse risk adjusted on disease classification (MDS without blast excess, HR: 0.35, p<0.0001), cytogenetics (intermediate, HR: 1.6, p=0.03; poor, HR: 1.8, p=0.002; AML, HR: 1.7, p=0.003; unknown, HR=1.6, p=0.07) and type of donor (unrelated, HR: 0.7, p=0.002), TCD increased significantly the relapse risk (HR: 1.5, p=0.005).
In the multivariable models for TRM adjusted on age (HR: 1.01, p=0.003), type of donor (unrelated, HR: 1.3, p=0.02), Karnofsky score (HR: 0.98, p<0.0001), platelet count at time of transplant (HR; 0.36/1 Giga, p=0.03), CMV serology (patient positive, HR: 1.3, p=0.005), TCD has no significant impact (HR: 0.99, p=0.97).
Conclusion: TCD decreased the risk of chronic GVHD and increased the risk of relapse confirming the potential graft-versus-MDS effect. The reduction of chronic GVHD do not translate into a decreased TRM and do not impact OS in patients who did not receive TCD while GRFS tended to be better in patients who received TCD. This study shows in a specific cohort of MDS with a relative long follow-up that TCD has major impact on outcome.
Forcade: Neovii: Other: Travel grant. Socié: Alexion Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal